
Optimized the vanadium electrolyte with sulfate-phosphoric
The sulfate-phosphoric mixed acid system electrolyte promotes the electrode reaction process, increases the current density, and reduces the resistance. This work systematically optimizes
Get Started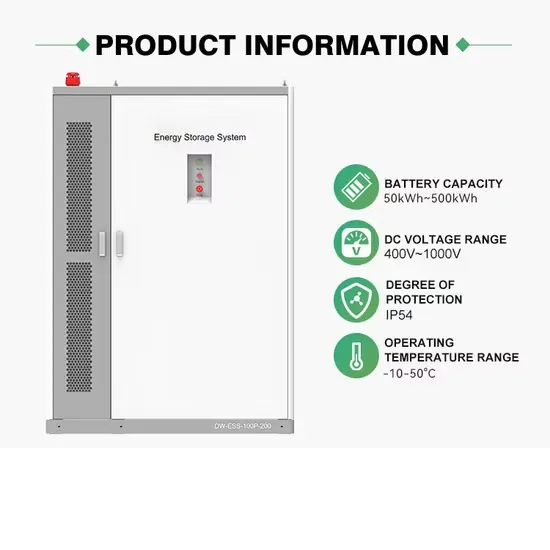
Understanding the Role of Battery Acid in Automotive
Jun 21, 2024 · Battery acid primarily refers to sulfuric acid, which is utilized in conventional lead-acid car batteries. This type of acid plays a critical role, as it facilitates the electrochemical
Get Started
How Lead-Acid Batteries Work
Feb 14, 2025 · Lead-acid batteries work by harnessing the chemical reactions between lead plates and sulfuric acid to store and release electrical energy.
Get Started
10.2 Batteries and Electrolytic Cells
The lead–acid battery is a common battery used to provide the starting power in virtually every automobile and marine engine on the market. Marine and car
Get Started
Revealing the role of phosphoric acid in all-vanadium redox flow
Jan 1, 2018 · The present work suggests the use of a mixed water-based electrolyte containing sulfuric and phosphoric acid for both negative and positive electrolytes of a vanadium redox
Get Started
Unveiling the Significance of Sulfuric Acid in Lead Acid Battery
Apr 11, 2025 · Sulfuric acid acts as the electrolyte, facilitating ion exchange between lead plates during charging and discharging. Its high acidity allows dissolution of sulfate ions (SO₄²⁻),
Get Started
Sulfuric Acid in Battery Manufacturing
Why Is Sulfuric Acid Essential for Lead-Acid Batteries? Sulfuric acid plays a crucial role in battery function due to its: High Ionic Conductivity – Enhances
Get Started
The Unseen Backbone of Battery Recycling: Sulfuric Acid''s
Jun 18, 2025 · As the world accelerates its transition to clean energy, sulfuric acid is quietly assuming a pivotal role in battery recycling and critical mineral recovery —key pillars of the
Get Started
Reasons why a battery contains acid
Jan 14, 2024 · So, in summary, lead plays a crucial role in the acidification of a battery. It reacts with sulfuric acid during discharge and then regenerates during charging, causing the battery
Get Started
What is Battery Acid and How Does it Work
Jan 14, 2024 · Battery acid, also known as sulfuric acid, is a highly corrosive liquid that plays a critical role in the functioning of batteries. To understand what battery acid is made up of and
Get Started
Novel electrolyte design for high-efficiency vanadium redox flow
Jul 15, 2025 · Improved broad temperature adaptability and energy density of vanadium redox flow battery based on sulfate-chloride mixed acid by optimizing the concentration of electrolyte
Get Started
Electrolyte tank costs are an overlooked factor in flow battery
Jan 3, 2025 · Quotes from globally distributed sulfuric acid storage tank manufacturers demonstrate that electrolyte tank costs are a substantial factor in flow battery development
Get Started
Revealing sulfuric acid concentration impact on
Apr 20, 2019 · The redox flow batteries (RFBs) play a crucial role among them due to many merits including unlimited capacity and power, long cycle life, environmental friendliness and high
Get Started
The role of phosphate additive in stabilization of sulphuric-acid
Sep 30, 2017 · Catholyte in all-vanadium redox-flow battery (VRFB) which consists of vanadium salts dissolved in sulphuric acid is known to be stabilized by phosphoric acid to slow down the
Get Started
Adjustment of Electrolyte Composition for
Oct 16, 2023 · Commercial electrolyte for vanadium flow batteries is modified by dilution with sulfuric and phosphoric acid so that series of electrolytes with
Get Started
Flow Cell for Simultaneous In Situ Analysis of Local
May 17, 2023 · Serving as both the electrolyte and the active material, sulfuric acid is a crucial component in all lead-acid batteries. It provides the medium for mass transfer and ionic charge
Get Started
Battery Acid Ph: Optimizing Electrolyte Concentration
Sep 24, 2024 · Sulfuric acid, with its fiery reputation, is a potent acid that acts as the electrolyte in batteries. This means it provides the conductive medium for the flow of electrical current. In
Get Started
Battery Acid: Name, Supphuric Acid, pH, and Role in Car Batteries
Battery acid is the liquid electrolyte inside a lead-acid battery. This liquid is a mixture of sulfuric acid (H₂SO₄) and water. It plays a vital role in storing and releasing electrical energy through
Get Started
Comprehensive Guide to Battery Acid: Its
Oct 3, 2024 · Battery acid, primarily composed of sulfuric acid (H₂SO₄), is a highly corrosive liquid used in certain types of batteries, particularly lead-acid
Get Started
Sulfuric Acid in Battery Manufacturing
Sulfuric acid plays a crucial role in battery function due to its: High Ionic Conductivity – Enhances electron flow between the battery plates. Efficient
Get Started
Why Is Sulfuric Acid Used in Car Batteries? Explained
May 13, 2025 · At the cathode, lead dioxide reacts with sulfuric acid and the electrons from the anode to form lead sulfate and water. This process effectively completes the circuit, allowing
Get Started
On the significance of sulphuric acid dissociation in the
Aug 1, 2020 · A recent asymptotic model for the operation of a vanadium redox flow battery (VRFB) is extended to include the dissociation of sulphuric acid—a bulk chemical reaction that
Get Started
The Vital Role of Sulfuric Acid in Battery Acid
By understanding the benefits of sulfuric acid in battery acid production, manufacturers can optimize their production processes and create high-quality
Get Started
Acid For Batteries: Types, Functions & Safety Guide
Jul 5, 2025 · What is sulfuric acid used for in batteries? Sulfuric acid is mainly used as the electrolyte in lead-acid batteries. It helps in the chemical reactions that produce electricity.
Get Started
The Vital Role of Sulfuric Acid in Battery Acid
In the production of lead-acid batteries, sulfuric acid plays a vital role as an electrolyte. The electrolyte is a chemical substance that facilitates the flow of
Get Started
Review of vanadium redox flow battery technology
Vanadium redox flow battery (VRFB) has a brilliant future in the field of large energy storage system (EES) due to its characteristics including fast response speed, large energy
Get Started
Revealing the Role of Phosphoric Acid in All-Vanadium Redox Flow
Aug 31, 2018 · Abstract The present work suggests the use of a mixed water-based electrolyte containing sulfuric and phosphoric acid for both negative and positive electrolytes of a
Get Started
Decoding the Electrolyte-Involved Chemical Reactions in Lead Acid Batteries
Apr 11, 2025 · Lead acid batteries generate electricity through electrolyte-driven chemical reactions. During discharge, sulfuric acid (H₂SO₄) reacts with lead plates, producing lead
Get Started
What is Battery Acid? Learn About its Composition and Uses
Jan 14, 2024 · Battery acid, also known as electrolyte, is a solution that is commonly found in lead-acid batteries. This acid is a vital component of the battery, as it plays a crucial role in its
Get Started
How Acid Powers Your Car Battery | ShunAuto
Apr 24, 2025 · Car batteries are fascinating components that power vehicles. The liquid in car batteries is typically a corrosive substance known as battery acid
Get Started
Comprehensive Guide to Battery Acid: Its
Oct 3, 2024 · Understanding Battery Acid: Types, Uses, and Safety Battery acid, primarily composed of sulfuric acid (H₂SO₄), is a highly corrosive liquid used
Get Started
Types of Battery Acid Used in Different Batteries
Jan 14, 2024 · Overall, sulfuric acid plays a crucial role in the functionality of lead-acid batteries, providing the necessary electrolyte for the battery cells. Its corrosive nature and strong
Get Started
6 FAQs about [The role of sulfuric acid in flow batteries]
How does sulfuric acid affect a car battery?
The sulfuric acid solution, known as the electrolyte, facilitates the flow of ions between the electrodes, enabling the chemical reactions that generate electricity. When a car battery is connected to a circuit, a chemical reaction occurs at both electrodes.
Why is sulfuric acid a good battery?
Sulfuric acid has a relatively low vapor pressure, meaning it does not easily evaporate. This property helps to prevent the loss of electrolyte from the battery, ensuring its longevity. Sulfuric acid has a high density, which contributes to the overall weight of the battery. This density also helps to maintain the battery’s structural integrity.
Why is sulfuric acid used in energy storage?
Sulfuric acid serves as the electrolyte in these batteries, facilitating the flow of electrons and thus allowing the battery to generate and store energy efficiently. One of the major advantages of using battery acid in energy storage is its ability to deliver high surges of electricity.
Why is sulfuric acid a good electrolyte?
Sulfuric acid acts as the electrolyte catalyst, enabling ion transfer between lead plates. It dissociates into H⁺ and SO₄²⁻ ions during discharge, facilitating electron flow through external circuits. Optimal specific gravity (1.22-1.28) ensures peak conductivity.
What is battery acid?
Battery acid, primarily composed of sulfuric acid (H₂SO₄), is a highly corrosive liquid used in certain types of batteries, particularly lead-acid batteries. This powerful acid plays a pivotal role in the batteries’ energy storage and ability to produce power on demand.
Is sulfuric acid a good electrolyte for car batteries?
The answer lies in a surprisingly simple yet powerful chemical: sulfuric acid. Sulfuric acid, with its distinctive pungent odor, might seem like an unlikely hero in the world of automotive technology. However, its unique properties make it the perfect electrolyte for lead-acid batteries, the most common type found in cars today.
Related Articles
-
 Environmental assessment requirements for liquid flow batteries for communication base stations
Environmental assessment requirements for liquid flow batteries for communication base stations
-
 10 billion flow batteries
10 billion flow batteries
-
 What are the photovoltaic power generation of flow batteries in Kiribati communication base stations
What are the photovoltaic power generation of flow batteries in Kiribati communication base stations
-
 The prospects of zinc-nickel flow batteries
The prospects of zinc-nickel flow batteries
-
 Approval of liquid flow batteries for communication base stations
Approval of liquid flow batteries for communication base stations
-
 Advantages and disadvantages of high-efficiency liquid flow batteries
Advantages and disadvantages of high-efficiency liquid flow batteries
-
 Commercialization of vanadium flow batteries
Commercialization of vanadium flow batteries
-
 Several types of flow batteries
Several types of flow batteries
-
 Industry standards for flow batteries
Industry standards for flow batteries
-
 Georgia Energy Storage Lead Acid Batteries
Georgia Energy Storage Lead Acid Batteries
Commercial & Industrial Solar Storage Market Growth
The global commercial and industrial solar energy storage battery market is experiencing unprecedented growth, with demand increasing by over 400% in the past three years. Large-scale battery storage solutions now account for approximately 45% of all new commercial solar installations worldwide. North America leads with 42% market share, driven by corporate sustainability goals and federal investment tax credits that reduce total system costs by 30-35%. Europe follows with 35% market share, where standardized industrial storage designs have cut installation timelines by 60% compared to custom solutions. Asia-Pacific represents the fastest-growing region at 50% CAGR, with manufacturing innovations reducing system prices by 20% annually. Emerging markets are adopting commercial storage for peak shaving and energy cost reduction, with typical payback periods of 3-6 years. Modern industrial installations now feature integrated systems with 50kWh to multi-megawatt capacity at costs below $500/kWh for complete energy solutions.
Solar Battery Innovations & Industrial Cost Benefits
Technological advancements are dramatically improving solar energy storage battery performance while reducing costs for commercial applications. Next-generation battery management systems maintain optimal performance with 50% less energy loss, extending battery lifespan to 20+ years. Standardized plug-and-play designs have reduced installation costs from $1,000/kW to $550/kW since 2022. Smart integration features now allow industrial systems to operate as virtual power plants, increasing business savings by 40% through time-of-use optimization and grid services. Safety innovations including multi-stage protection and thermal management systems have reduced insurance premiums by 30% for commercial storage installations. New modular designs enable capacity expansion through simple battery additions at just $450/kWh for incremental storage. These innovations have improved ROI significantly, with commercial projects typically achieving payback in 4-7 years depending on local electricity rates and incentive programs. Recent pricing trends show standard industrial systems (50-100kWh) starting at $25,000 and premium systems (200-500kWh) from $100,000, with flexible financing options available for businesses.


